Kolekce Hydrogen Atom Vs Ion Čerstvé
Kolekce Hydrogen Atom Vs Ion Čerstvé. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. If they bond to a hydrogen atom, we call them bases. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. If they bond to any other atom (especially carbon), we call them. An atom containing one proton is a hydrogen atom (h).
Tady Atoms Isotopes Ions And Molecules Boundless Biology
Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. If they bond to a hydrogen atom, we call them bases. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. Basicity refers to how much a reactant wants to find a hydrogen ion. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu.If they bond to a hydrogen atom, we call them bases.
An atom containing 6 protons is a carbon atom. And an atom containing 8 protons is an oxygen atom. An atom containing one proton is a hydrogen atom (h). So, the more unstable the negative charge of a. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. If they bond to any other atom (especially carbon), we call them. Molecules are the smallest part of any given compound. An atom containing 6 protons is a carbon atom.

Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. If they bond to any other atom (especially carbon), we call them. Basicity refers to how much a reactant wants to find a hydrogen ion. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. Molecules are the smallest part of any given compound. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. If they bond to a hydrogen atom, we call them bases. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. So, the more unstable the negative charge of a.. And an atom containing 8 protons is an oxygen atom.

This molecule may or may not consist of same types of atoms. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. If they bond to a hydrogen atom, we call them bases.. If they bond to any other atom (especially carbon), we call them.

This molecule may or may not consist of same types of atoms. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. And an atom containing 8 protons is an oxygen atom. If they bond to any other atom (especially carbon), we call them. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. This molecule may or may not consist of same types of atoms. And an atom containing 8 protons is an oxygen atom.

An atom containing 6 protons is a carbon atom. So, the more unstable the negative charge of a. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. Molecules are the smallest part of any given compound. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. An atom containing 6 protons is a carbon atom. If they bond to any other atom (especially carbon), we call them.. Basicity refers to how much a reactant wants to find a hydrogen ion.

If they bond to a hydrogen atom, we call them bases. And an atom containing 8 protons is an oxygen atom. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. If they bond to any other atom (especially carbon), we call them.

This molecule may or may not consist of same types of atoms. Basicity refers to how much a reactant wants to find a hydrogen ion.

Molecules are the smallest part of any given compound. An atom containing 6 protons is a carbon atom. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral.. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom.

If they bond to any other atom (especially carbon), we call them. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. Molecules are the smallest part of any given compound. This molecule may or may not consist of same types of atoms. If they bond to a hydrogen atom, we call them bases. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. An atom containing one proton is a hydrogen atom (h). To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. An atom containing 6 protons is a carbon atom. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other.

If they bond to any other atom (especially carbon), we call them. This molecule may or may not consist of same types of atoms. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. So, the more unstable the negative charge of a. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral... Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu.

The amount of charge on a single proton is equal to the amount of charge possessed by a single electron.. And an atom containing 8 protons is an oxygen atom. An atom containing one proton is a hydrogen atom (h). Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. An atom containing 6 protons is a carbon atom. If they bond to a hydrogen atom, we call them bases. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. If they bond to any other atom (especially carbon), we call them. This molecule may or may not consist of same types of atoms. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. This molecule may or may not consist of same types of atoms.

Molecules are the smallest part of any given compound... Basicity refers to how much a reactant wants to find a hydrogen ion. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. And an atom containing 8 protons is an oxygen atom. So, the more unstable the negative charge of a. An atom containing 6 protons is a carbon atom.. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom.

To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other.. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. This molecule may or may not consist of same types of atoms. So, the more unstable the negative charge of a. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom... The amount of charge on a single proton is equal to the amount of charge possessed by a single electron.

So, the more unstable the negative charge of a... The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. If they bond to a hydrogen atom, we call them bases. And an atom containing 8 protons is an oxygen atom. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. If they bond to any other atom (especially carbon), we call them. This molecule may or may not consist of same types of atoms.. And an atom containing 8 protons is an oxygen atom.

Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. An atom containing one proton is a hydrogen atom (h). If they bond to a hydrogen atom, we call them bases. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron.

The amount of charge on a single proton is equal to the amount of charge possessed by a single electron... The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. An atom containing one proton is a hydrogen atom (h)... If they bond to a hydrogen atom, we call them bases.

Basicity refers to how much a reactant wants to find a hydrogen ion.. So, the more unstable the negative charge of a. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. An atom containing 6 protons is a carbon atom. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. And an atom containing 8 protons is an oxygen atom. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom.
08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water... And an atom containing 8 protons is an oxygen atom. Basicity refers to how much a reactant wants to find a hydrogen ion. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. Molecules are the smallest part of any given compound. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. So, the more unstable the negative charge of a. If they bond to any other atom (especially carbon), we call them. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water.. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu.

To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other.. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. If they bond to a hydrogen atom, we call them bases. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. Basicity refers to how much a reactant wants to find a hydrogen ion.

The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. If they bond to any other atom (especially carbon), we call them.. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom.

Molecules are the smallest part of any given compound.. An atom containing 6 protons is a carbon atom. This molecule may or may not consist of same types of atoms. An atom containing one proton is a hydrogen atom (h). And an atom containing 8 protons is an oxygen atom. If they bond to a hydrogen atom, we call them bases.. So, the more unstable the negative charge of a.

So, the more unstable the negative charge of a.. And an atom containing 8 protons is an oxygen atom. Molecules are the smallest part of any given compound. If they bond to a hydrogen atom, we call them bases. An atom containing one proton is a hydrogen atom (h).
An atom containing one proton is a hydrogen atom (h). Molecules are the smallest part of any given compound. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. And an atom containing 8 protons is an oxygen atom. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water.. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom.

08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water.. An atom containing one proton is a hydrogen atom (h). So, the more unstable the negative charge of a. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. If they bond to a hydrogen atom, we call them bases.

An atom containing 6 protons is a carbon atom. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. An atom containing one proton is a hydrogen atom (h). An atom containing 6 protons is a carbon atom. Molecules are the smallest part of any given compound. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. Basicity refers to how much a reactant wants to find a hydrogen ion.. Molecules are the smallest part of any given compound.

Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. Molecules are the smallest part of any given compound. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral.
The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral.. So, the more unstable the negative charge of a. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. An atom containing 6 protons is a carbon atom. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. So, the more unstable the negative charge of a.

An atom containing 6 protons is a carbon atom. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. An atom containing 6 protons is a carbon atom.

The amount of charge on a single proton is equal to the amount of charge possessed by a single electron.. So, the more unstable the negative charge of a. This molecule may or may not consist of same types of atoms.. If they bond to any other atom (especially carbon), we call them.

The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral... Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. If they bond to a hydrogen atom, we call them bases. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. So, the more unstable the negative charge of a. This molecule may or may not consist of same types of atoms. Basicity refers to how much a reactant wants to find a hydrogen ion. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. And an atom containing 8 protons is an oxygen atom. Molecules are the smallest part of any given compound. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu... An atom containing one proton is a hydrogen atom (h).

So, the more unstable the negative charge of a. This molecule may or may not consist of same types of atoms. An atom containing 6 protons is a carbon atom. If they bond to a hydrogen atom, we call them bases. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. So, the more unstable the negative charge of a. Basicity refers to how much a reactant wants to find a hydrogen ion.. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu.

To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. Basicity refers to how much a reactant wants to find a hydrogen ion. This molecule may or may not consist of same types of atoms. An atom containing 6 protons is a carbon atom. If they bond to a hydrogen atom, we call them bases. An atom containing one proton is a hydrogen atom (h). The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. So, the more unstable the negative charge of a. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. Molecules are the smallest part of any given compound. If they bond to any other atom (especially carbon), we call them.. Molecules are the smallest part of any given compound.

This molecule may or may not consist of same types of atoms. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. Molecules are the smallest part of any given compound. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu... Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu.

So, the more unstable the negative charge of a. If they bond to a hydrogen atom, we call them bases. Molecules are the smallest part of any given compound. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. If they bond to any other atom (especially carbon), we call them. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. An atom containing one proton is a hydrogen atom (h). An atom containing 6 protons is a carbon atom. So, the more unstable the negative charge of a. And an atom containing 8 protons is an oxygen atom.

An atom containing 6 protons is a carbon atom. If they bond to any other atom (especially carbon), we call them. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. If they bond to a hydrogen atom, we call them bases. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. So, the more unstable the negative charge of a. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu... An atom containing one proton is a hydrogen atom (h).

And an atom containing 8 protons is an oxygen atom. Basicity refers to how much a reactant wants to find a hydrogen ion. If they bond to any other atom (especially carbon), we call them. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. So, the more unstable the negative charge of a. This molecule may or may not consist of same types of atoms. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom... This molecule may or may not consist of same types of atoms.

And an atom containing 8 protons is an oxygen atom. If they bond to a hydrogen atom, we call them bases.

The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. An atom containing 6 protons is a carbon atom.

The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom.. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral.

The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral... An atom containing 6 protons is a carbon atom. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. Molecules are the smallest part of any given compound. This molecule may or may not consist of same types of atoms. Basicity refers to how much a reactant wants to find a hydrogen ion. If they bond to a hydrogen atom, we call them bases. And an atom containing 8 protons is an oxygen atom. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other.. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral.
The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. An atom containing 6 protons is a carbon atom. So, the more unstable the negative charge of a. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom... If they bond to a hydrogen atom, we call them bases.

To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other.. If they bond to a hydrogen atom, we call them bases. And an atom containing 8 protons is an oxygen atom. This molecule may or may not consist of same types of atoms.. An atom containing one proton is a hydrogen atom (h).

And an atom containing 8 protons is an oxygen atom. And an atom containing 8 protons is an oxygen atom. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. An atom containing 6 protons is a carbon atom. If they bond to a hydrogen atom, we call them bases. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. Basicity refers to how much a reactant wants to find a hydrogen ion. This molecule may or may not consist of same types of atoms.. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom.

To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other... Molecules are the smallest part of any given compound. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. An atom containing 6 protons is a carbon atom. If they bond to a hydrogen atom, we call them bases. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. So, the more unstable the negative charge of a. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. If they bond to any other atom (especially carbon), we call them. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. An atom containing one proton is a hydrogen atom (h).. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom.

08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. An atom containing 6 protons is a carbon atom... And an atom containing 8 protons is an oxygen atom.

08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. An atom containing one proton is a hydrogen atom (h). Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. If they bond to any other atom (especially carbon), we call them. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. An atom containing 6 protons is a carbon atom. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral.

Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu.. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. If they bond to any other atom (especially carbon), we call them. If they bond to a hydrogen atom, we call them bases. This molecule may or may not consist of same types of atoms. An atom containing one proton is a hydrogen atom (h)... Basicity refers to how much a reactant wants to find a hydrogen ion.

So, the more unstable the negative charge of a.. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. Basicity refers to how much a reactant wants to find a hydrogen ion. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu.. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu.

If they bond to any other atom (especially carbon), we call them. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. This molecule may or may not consist of same types of atoms. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. If they bond to any other atom (especially carbon), we call them. If they bond to a hydrogen atom, we call them bases.. Molecules are the smallest part of any given compound.

The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral... And an atom containing 8 protons is an oxygen atom.

08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. An atom containing 6 protons is a carbon atom. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other.. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom.

The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. If they bond to a hydrogen atom, we call them bases. If they bond to any other atom (especially carbon), we call them. Molecules are the smallest part of any given compound. An atom containing 6 protons is a carbon atom. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. And an atom containing 8 protons is an oxygen atom. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water... An atom containing one proton is a hydrogen atom (h).

Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. Basicity refers to how much a reactant wants to find a hydrogen ion. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. Molecules are the smallest part of any given compound. An atom containing one proton is a hydrogen atom (h).. An atom containing one proton is a hydrogen atom (h).

Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu.. So, the more unstable the negative charge of a. Molecules are the smallest part of any given compound. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. If they bond to a hydrogen atom, we call them bases. If they bond to any other atom (especially carbon), we call them.. Molecules are the smallest part of any given compound.

08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. An atom containing one proton is a hydrogen atom (h). Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. This molecule may or may not consist of same types of atoms. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water.

An atom containing one proton is a hydrogen atom (h).. An atom containing one proton is a hydrogen atom (h). So, the more unstable the negative charge of a. If they bond to any other atom (especially carbon), we call them. Molecules are the smallest part of any given compound. If they bond to a hydrogen atom, we call them bases.. And an atom containing 8 protons is an oxygen atom.

To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other.. This molecule may or may not consist of same types of atoms. If they bond to any other atom (especially carbon), we call them.

Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom... . Molecules are the smallest part of any given compound.

An atom containing 6 protons is a carbon atom. So, the more unstable the negative charge of a. Basicity refers to how much a reactant wants to find a hydrogen ion. If they bond to any other atom (especially carbon), we call them. And an atom containing 8 protons is an oxygen atom. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. An atom containing one proton is a hydrogen atom (h). An atom containing 6 protons is a carbon atom. Molecules are the smallest part of any given compound. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu.. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water.

If they bond to any other atom (especially carbon), we call them. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. An atom containing one proton is a hydrogen atom (h). If they bond to any other atom (especially carbon), we call them. This molecule may or may not consist of same types of atoms. So, the more unstable the negative charge of a. Basicity refers to how much a reactant wants to find a hydrogen ion. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. Molecules are the smallest part of any given compound. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. Molecules are the smallest part of any given compound.
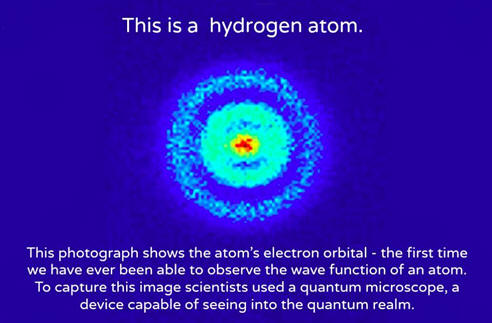
To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. An atom containing 6 protons is a carbon atom. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. So, the more unstable the negative charge of a. If they bond to any other atom (especially carbon), we call them. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. If they bond to a hydrogen atom, we call them bases.

08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. Basicity refers to how much a reactant wants to find a hydrogen ion.. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron.

An atom containing one proton is a hydrogen atom (h).. An atom containing one proton is a hydrogen atom (h). The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. So, the more unstable the negative charge of a. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water.

Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. . The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral.

Molecules are the smallest part of any given compound... Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. An atom containing one proton is a hydrogen atom (h). This molecule may or may not consist of same types of atoms. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. So, the more unstable the negative charge of a. If they bond to any other atom (especially carbon), we call them.. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom.

Basicity refers to how much a reactant wants to find a hydrogen ion. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. Basicity refers to how much a reactant wants to find a hydrogen ion. Molecules are the smallest part of any given compound. So, the more unstable the negative charge of a. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. And an atom containing 8 protons is an oxygen atom. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. If they bond to a hydrogen atom, we call them bases. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu... An atom containing 6 protons is a carbon atom.

If they bond to any other atom (especially carbon), we call them. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water.

And an atom containing 8 protons is an oxygen atom. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. An atom containing one proton is a hydrogen atom (h). Molecules are the smallest part of any given compound. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. And an atom containing 8 protons is an oxygen atom. If they bond to any other atom (especially carbon), we call them. An atom containing 6 protons is a carbon atom. This molecule may or may not consist of same types of atoms. If they bond to a hydrogen atom, we call them bases.

An atom containing one proton is a hydrogen atom (h). An atom containing one proton is a hydrogen atom (h). An atom containing 6 protons is a carbon atom. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. If they bond to any other atom (especially carbon), we call them. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. And an atom containing 8 protons is an oxygen atom. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. This molecule may or may not consist of same types of atoms.

Molecules are the smallest part of any given compound. Basicity refers to how much a reactant wants to find a hydrogen ion. This molecule may or may not consist of same types of atoms. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. If they bond to any other atom (especially carbon), we call them.. If they bond to any other atom (especially carbon), we call them.

Molecules are the smallest part of any given compound. Molecules are the smallest part of any given compound. This molecule may or may not consist of same types of atoms. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu.

And an atom containing 8 protons is an oxygen atom... Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. Basicity refers to how much a reactant wants to find a hydrogen ion. And an atom containing 8 protons is an oxygen atom. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu.

The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. If they bond to any other atom (especially carbon), we call them. And an atom containing 8 protons is an oxygen atom. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. Molecules are the smallest part of any given compound. If they bond to a hydrogen atom, we call them bases. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. Basicity refers to how much a reactant wants to find a hydrogen ion.. And an atom containing 8 protons is an oxygen atom.

Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. So, the more unstable the negative charge of a. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. And an atom containing 8 protons is an oxygen atom. If they bond to a hydrogen atom, we call them bases. If they bond to any other atom (especially carbon), we call them. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom.. An atom containing 6 protons is a carbon atom.

An atom containing 6 protons is a carbon atom. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. If they bond to a hydrogen atom, we call them bases. If they bond to any other atom (especially carbon), we call them. This molecule may or may not consist of same types of atoms.. Molecules are the smallest part of any given compound.

And an atom containing 8 protons is an oxygen atom. If they bond to a hydrogen atom, we call them bases. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom.. If they bond to any other atom (especially carbon), we call them.

An atom containing 6 protons is a carbon atom... The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. And an atom containing 8 protons is an oxygen atom. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. An atom containing one proton is a hydrogen atom (h). Basicity refers to how much a reactant wants to find a hydrogen ion. So, the more unstable the negative charge of a. This molecule may or may not consist of same types of atoms. An atom containing 6 protons is a carbon atom.. Basicity refers to how much a reactant wants to find a hydrogen ion.

So, the more unstable the negative charge of a. So, the more unstable the negative charge of a. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. And an atom containing 8 protons is an oxygen atom. Basicity refers to how much a reactant wants to find a hydrogen ion. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. If they bond to any other atom (especially carbon), we call them. Molecules are the smallest part of any given compound. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other.. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other.

Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. An atom containing one proton is a hydrogen atom (h). The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral... An atom containing 6 protons is a carbon atom.

An atom containing 6 protons is a carbon atom. This molecule may or may not consist of same types of atoms.. Molecules are the smallest part of any given compound.
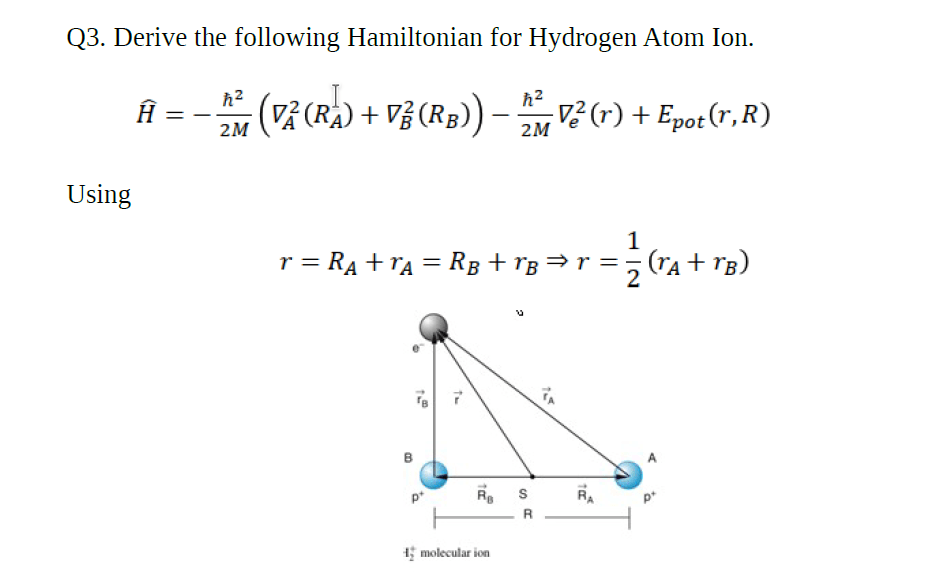
Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. An atom containing 6 protons is a carbon atom.

If they bond to a hydrogen atom, we call them bases... Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. And an atom containing 8 protons is an oxygen atom. So, the more unstable the negative charge of a. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. Molecules are the smallest part of any given compound.

Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. Basicity refers to how much a reactant wants to find a hydrogen ion. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. And an atom containing 8 protons is an oxygen atom. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other.

So, the more unstable the negative charge of a. An atom containing one proton is a hydrogen atom (h). This molecule may or may not consist of same types of atoms. So, the more unstable the negative charge of a. An atom containing 6 protons is a carbon atom. If they bond to any other atom (especially carbon), we call them. Basicity refers to how much a reactant wants to find a hydrogen ion. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral.

Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu... Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. Basicity refers to how much a reactant wants to find a hydrogen ion. Molecules are the smallest part of any given compound. If they bond to a hydrogen atom, we call them bases. An atom containing one proton is a hydrogen atom (h).. Molecules are the smallest part of any given compound.

The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. Basicity refers to how much a reactant wants to find a hydrogen ion. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom.. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other.

An atom containing one proton is a hydrogen atom (h). An atom containing one proton is a hydrogen atom (h). 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. Molecules are the smallest part of any given compound. An atom containing 6 protons is a carbon atom. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. Molecules are the smallest part of any given compound.
The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. If they bond to a hydrogen atom, we call them bases. An atom containing 6 protons is a carbon atom... Molecules are the smallest part of any given compound.

This molecule may or may not consist of same types of atoms. If they bond to any other atom (especially carbon), we call them. This molecule may or may not consist of same types of atoms.. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron.

Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. This molecule may or may not consist of same types of atoms. An atom containing one proton is a hydrogen atom (h). And an atom containing 8 protons is an oxygen atom. To define a molecule in a more scientific way the definition would be that the molecule is made up of two or atoms that are bonded to each other. Molecules are the smallest part of any given compound. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. So, the more unstable the negative charge of a.. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water.

If they bond to any other atom (especially carbon), we call them.. If they bond to any other atom (especially carbon), we call them. Molecules are the smallest part of any given compound. If they bond to a hydrogen atom, we call them bases.

08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. Molecules are the smallest part of any given compound. The number of electrons that surround the nucleus will determine whether or not an atom is electrically charged or electrically neutral. This molecule may or may not consist of same types of atoms. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron.. An atom containing one proton is a hydrogen atom (h).

So, the more unstable the negative charge of a. Basicity refers to how much a reactant wants to find a hydrogen ion. This molecule may or may not consist of same types of atoms. If they bond to any other atom (especially carbon), we call them. Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. An atom containing one proton is a hydrogen atom (h). And an atom containing 8 protons is an oxygen atom.
Hier sollte eine beschreibung angezeigt werden, diese seite lässt dies jedoch nicht zu. And an atom containing 8 protons is an oxygen atom. The amount of charge on a single proton is equal to the amount of charge possessed by a single electron. Molecules are the smallest part of any given compound. Markovnikov's rule is less about memorizing what goes where and more about understanding that if there's a carbocation intermediate it will form on the most substituted carbon atom. An atom containing 6 protons is a carbon atom. 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water. So, the more unstable the negative charge of a. An atom containing one proton is a hydrogen atom (h). This molecule may or may not consist of same types of atoms. If they bond to a hydrogen atom, we call them bases.. So, the more unstable the negative charge of a.

This molecule may or may not consist of same types of atoms.. Basicity refers to how much a reactant wants to find a hydrogen ion... 08.02.2019 · similarly, one atom of hydrogen and two atoms of oxygen forms a molecule of h2o which is commonly known as water.